The development of Covid-19 vaccines was an important step in controlling the pandemic. Several national regulatory authorities in Latin America aimed to harmonise regulation. As Elize Massard da Fonseca (Latin America and Caribbean Centre, LSE) explains, there will be more literature on policy regulation exploring new dimensions on this matter.
International inequalities in vaccine access and the individual-level factors that predict vaccine acceptance have been a subject of analysis in the academic world. However, there has been less attention when it comes to discussing regulation. What did the authorities in Latin America do to ensure that Covid-19 vaccines were safe and readily available? How did they respond to economic and political pressures to rapidly approve these products?
These questions matter because pharmaceutical regulation is a crucial state function during moments of a health crisis (e.g., the HIV/AIDS and Ebola outbreaks), which can influence the speed and availability of products. Furthermore, strengthening regulatory systems has been a recent priority for many Latin American countries.
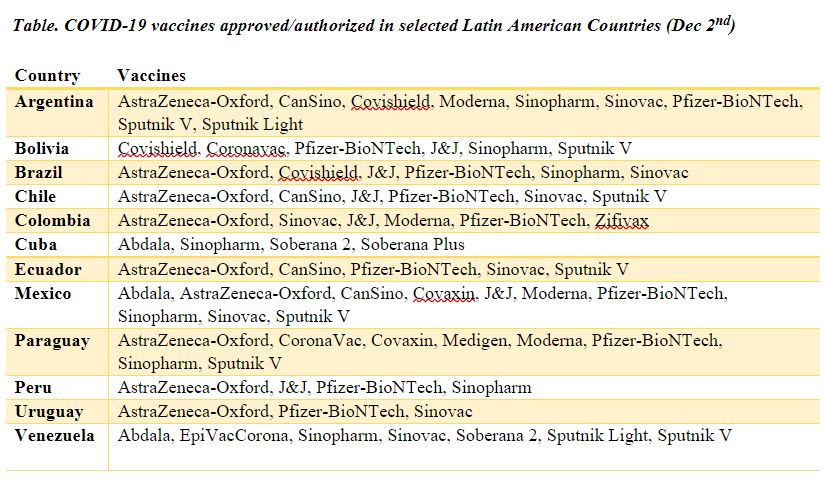
The development of Covid-19 vaccines was an important step in controlling the pandemic. As soon as they were approved in the US and Europe, Latin American governments followed suit. However, they approved different vaccines simultaneously, as shown in the table below.
Who regulates COVID-19 vaccines?
Vaccine developers submit a dossier to National Regulatory Authorities (NRAs) requesting clinical trials and/or marketing approval. Therefore, the first step is taken by the industry, and developers choose where they intend to conduct trial studies or commercialise their products.
Over the past three decades, the Pan American Health Organization (PAHO) has promoted efforts to strengthen regulatory systems and harmonise pharmaceutical regulatory norms in Latin America to reduce fragmentation, improve quality standards, and facilitate trade between counties. More recently, PAHO has assessed NRAs to better understand their regulatory functions. Agencies such as Anvisa in Brazil, Anmat in Argentina, and Cofepris in Mexico were listed as references for the region due to their functionality and expertise in regulatory and oversight capacities.
What should regulatory agencies do to streamline clinical trials and expedite approval?
In mid-2020, the Gates Foundation published an article that identified 50 pathways that can be applied to accelerate the scientific assessment and regulatory approval of biomedical products during public health crises. These are based on the procedures employed by stringent NRAs in Japan, the United States, the European Union and the World Health Organization. They include a rolling review in which scientific advice and presubmission meetings take place between developers and regulators to expedite regulatory assessment; Emergency Use Authorisation to authorise, with specific caveats, the use of biomedicals that are still under trial during the period of emergency.
Several Latin American regulatory authorities are part of international networks and initiatives that aim to harmonise regulation. These networks were crucial in facilitating the exchange of information and determining best practices during the pandemic. For instance, the Pharmaceutical Inspection Co-operation Scheme (PIC/S) promotes common standards of Good Manufacturing Process (GMP). This mechanism ensures that pharmaceuticals are manufactured according to specific quality standards. Instead of conducting their own GMP inspections, members of this scheme can rely on those operated by other members. This accelerates approval processes and avoids duplicate checks.
How can variations in COVID-19 vaccine regulation be explained?
Our team is initiating research to explore differences and regulatory choices in Latin American countries. The literature on the politics of regulation invites us to consider two dimensions.
First, it is important to consider how foreign regulatory norms, such as those proposed by Gates Foundation, are translated into local contexts. According to Covadonga Meseguer (2005), a leading scholar in the field of regulation and former professor at the LSE, learning is a voluntary act and requires a change of beliefs based on others’ experiences.
Therefore, when national regulatory authorities decide to rely on the WHO or international guidelines issued by other bodies or join international harmonisation forums, they do so voluntarily. When they are persuaded that these are credible regulatory pathways, regulators embrace these rules and translate them into their practices. Learning is driven by problem-solving and a search for regulatory solutions. Emulation, however, is driven by motivations that do not involve reflection on causal pathways from policies to outcomes. As Meseguer explains, “policy emulation is a ‘blind’ action in that it does not entail enhanced reflection about the mapping from policies to outcomes.” This is known as “symbolic imitation.” Therefore, in emulation, unlike learning, there is a lack of understanding of technical concepts. For example, emulation occurs when a politician sees a strategy being implemented in other countries and decides to adopt it in their own country without carefully considering its consequences. The difference between emulation and learning is significant because governments may adopt such international regulatory standards for different reasons.
Second, health policy scholars must seriously consider local political economies (Fonseca et al., 2022). The regulatory review of the Covid-19 vaccine candidates inevitably occurred under intense economic and political pressure. Historically, the literature on the political economy of regulation has defined the relationship between regulators and non-state actors through the lens of capture theory. This began with George Stigler, who outlined a model in which rent-seeking and industry influence the regulatory process. Indeed, different stakeholders may exert pressure and tentative influence on vaccine regulation; however, this does not necessarily translate into capture.
As pharmaceutical regulation deals with rules that govern market authorisation, and in the case of public health emergencies, in conditions of significant scientific uncertainty and urgency, the relationship between businesses and governments can be based on the mutually beneficial exchange of information rather than the exertion of pressure, as capture theory posits. Transmitting information becomes crucial as regulators need access to knowledge, for example, to understand the bottlenecks of streamlined clinical trials. The industry needs guidance about the streamlined formulation of vaccines.
What happens with the politicians that delegate regulatory tasks to these authorities? A 2021 report from the PAHO broadly hypotheses that “authorities with more administrative and technical independence are better equipped to fulfil regulatory functions well”.. Regulation scholarship portrays this relationship using the principle-agent framework. Regulatory authorities are classed as independent when they have certain institutional features that protect their decision-making processes from political actors. The FDA in the United States is an excellent example as it is mandated by the US Congress to regulate pharmaceuticals.
Although the literature on regulation in the Global South is growing, little is known about how these agencies, particularly those that deal with licenses and product approval, react to their principles. The Covid-19 pandemic provided an ideal case study to investigate delegation in the sector because regulators have faced intense pressure from politicians to regulate access to vaccines, and there will be other public health threats in the future.
Notes:
• The views expressed here are of the author rather than the Centre or the LSE
• Please read our Comments Policy before commenting
• Banner image: Vaccination process against COVID-19 in Guatemala City/ Byron Ortiz (Shutterstock)





